Abstract
Introduction/Background: Although randomized controlled trials are considered the gold standard of evidence for safety and efficacy of a drug, they are subject to several limitations such as difficulty in recruiting patients with rare diseases or randomization of patients to studies with treatments that lack an established standard of care owing to efficacy or toxicity concerns. RWE can help overcome these challenges. RWE collects patient data from historical trials or retrospective observational studies and compares or contextualizes these data to experimental treatments by using proper statistical adjustments.
The 21st Century Cures Act inspired the framework for the US FDA to use RWE to assess product effectiveness of approved drugs to support regulatory decision-making. Consequently, RWE is increasingly used in New Drug Applications (NDAs) and Biologics License Applications (BLAs) for hematologic malignancies, and findings suggest that submissions using RWE may receive faster approval. However, data are lacking on adoption of RWE for regulatory submissions and its use in regulatory decision-making. Here, we outline RWE utilization and limitations of regulatory submissions for hematologic malignancy therapeutics, which received accelerated or full approval in the US.
Methods: Between 2017 and 2021, all NDA and BLA approvals from the Division of Hematological Malignancies were reviewed for hematology/oncology drugs or indications. The FDA Oncology Drug Advisory Committee (ODAC) briefing and/or multidisciplinary review documents were evaluated to identify the drug approvals using RWE. This assessment was performed by 4 independent reviewers who evaluated FDA comments regarding RWE. RWE sources, study methodology, drug indications, pivotal trial information, statistical analyses, and results were summarized.
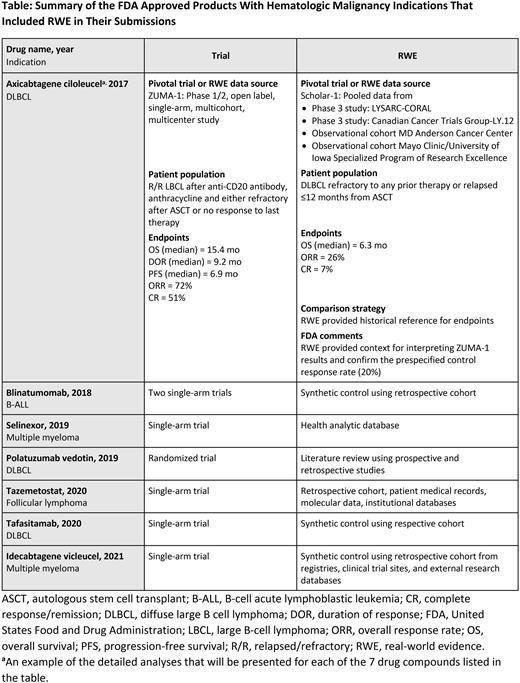
Results: A total of 74 hematologic malignancy approvals were identified, of which 7 (9.4%) provided RWE that supported regulatory approval. Detailed analyses of the FDA ODAC briefing and/or multidisciplinary review documents for these 7 drug compounds including comparison of trial and RWE data source, patient population, clinical endpoints and outcomes, comparison strategy, and FDA comments are shown in the Table.
The FDA identified several key considerations for RWE use for drug applications based on single-arm studies. These included pivotal trials that demonstrated a high response or large treatment effect, an improved drug toxicity profile, or a novel drug mechanism of action.
The most common RWE sources included retrospective observational studies from medical records, data from other clinical trials, commercial electronic health record databases, and literature reviews. The most common hematologic indications for RWE submissions were relapsed/refractory (R/R) large B-cell lymphoma and R/R multiple myeloma.
The most common endpoints for RWE included presence of measurable residual disease, complete response rate, and duration of response. In contrast, the most common endpoints in pivotal trials included survival endpoints (eg, progression-free survival). The most common statistical methods used to adjust for baseline imbalances included propensity-score matching and inverse-probability of treatment weighting.
The FDA identified several areas that may compromise the reliability of RWE: lack of early engagement and discussion of the study design with the FDA, absence of a prespecified statistical analysis plan or protocol, lack of transparency in data collection methods, dissimilar inclusion/exclusion criteria, dissimilar study endpoints, and an inconsistent method or frequency of outcome assessments. Comparator arms often missed baseline covariates, had selection bias, and used different outcome assessment criteria.
Conclusion: Good data quality, comparable patient populations, consistent endpoints, prespecified protocols, and the establishment of statistical analysis plans before the start of a study may be the most important factors in the FDA's approval of RWE regulatory submissions. Although the use of RWE to define a direct comparator was limited by flaws in study design and statistical analyses, RWE can be used to establish historical outcomes for patients with currently available treatment.
Disclosures
Yue:BeiGene USA, Inc.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Greenbaum:BeiGene: Current Employment, Current equity holder in publicly-traded company; ICON Plc: Ended employment in the past 24 months. Zhu:BeiGene USA, Inc.: Current Employment, Current equity holder in publicly-traded company. Weng:BeiGene: Current Employment; Medtronic: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Du:BeiGene: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal